Geiger Counter Circuits and Radioactivity
by Lewis Loflin
Here we will look into Geiger counters. This section looks at electronics involving Geiger tubes, power supplies, radiation basics, etc. My purchase of a Geiger counter, which we will discuss, I'll use this as a standard for the rest of the projects involved.
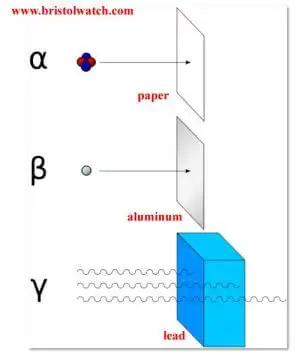
What is called nuclear radiation is both electromagnetic radiation and atomic particles. The penetrating power depends on both energy levels and atomic mass. Fig. 1 illustrates these properties. Alpha particles are a helium nucleus stripped of electrons with an atomic mass of 4 and a charge of +2. They are slow and can be stopped by a few sheets of paper, they are highly ionizing - they can cause tissue and cell damage.
Next are negatively charged Beta particles - an electron. Beta particles have little mass and are far more penetrating than Alpha particles and can be stopped by a sheet of aluminum or a few inches of wood. Energy levels vary - higher energy levels the more penetrating, but have a lower ionizing potential than Alpha particles.
Gamma rays are electromagnetic radiation like X-Rays, but more powerful I think - depends son energy level. They are the more penetrating, but least ionizing. They can be stopped by several inches of lead.
For more on this subject see Getting Real About Radiation Myths and Hazards.
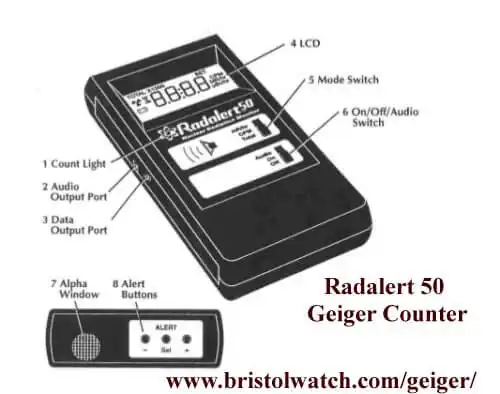
Fig. 2 Radalert 50TM Geiger Counter
Fig. 2 shows my Geiger counter a Radalert50TM that can detect Alpha, Beta, and Gamma radiation - not all Geiger counters can detect Alpha particles. Note the Alpha window in the illustration. The low-penetrating power of Alpha particles prevents them from passing through metal and class shells to trigger a discharge in the tube.

Fig. 3 LMD712 Geiger-Mueller Tube.
Fig. 3 is the LND712 tube used in my Geiger counter. A thin mica window will allow Alpha particles to pass into the tube.
For more in Geiger-Mueller tubes see Introduction to Geiger-Mueller Counters and Electronics

Fig. 4 Smoke detector radioactive alpha source.
Using a Geiger Counter
When the Geiger counter is turned on background radiation varies from 9 to 21 CPM or counts per minute. This is normal as cosmic rays, radon in the atmosphere, and various radioisotopes created random clicks.
The most common radioactive source in the home is a common smoke detector. It uses a small amount of Americium-241 an intense Alpha emitter. Americium-241 is produced in an atomic reactor in the same way plutonium is. As shown in the video a few sheets of paper stops the Alpha particles and normally adds nothing to background radiation.

Fig. 5 Weston Model 614 light level meter
has highly radioactive glass lens.
Click for larger picture.
After testing my Geiger counter on a junk smoke detector I went around the house checking radioactivity of my rock and mineral collection. No luck. I then went and check some of the early electronics in my collection and was stunned when a checked an old Weston Model 614 light level meter. Not only was there intense radiation through the Alpha window, but radiation penetrated the side of the case and the stainless steel Geiger tube shell. A few sheets of paper had little effect as shown in the video.
The radiation was coming from the selenium photocell section. Selenium was used in photovoltaic cells prior to the use of silicon after the 1940s. These meters were manufactured in the 1930s on according to literature.
There are no radioactive isotopes of selenium found in nature so that left the glass lens. Turns out prior to the 1960s glass lenses used up to 30% thorium oxide, which is 80-90% thorium. This also ended up in a lot of Kodak cameras.
Thorium itself isn't that radioactive, but its decay products are very radioactive. Thorium is a gray, heavy metal often mined with rare earth metals. It was also used mig welding rods and Coleman lamp mantels. Today thorium is no longer used in most applications and has little commercial use. It can be converted to nuclear fuel in an atomic reactor and its waste products are not as problematic as uranium fission products.
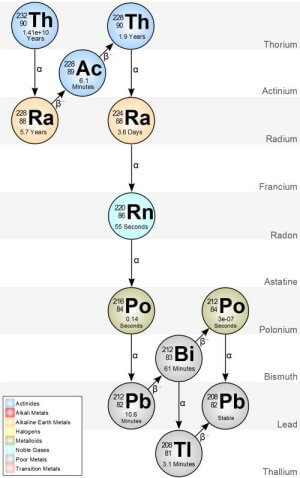
Note that radioactive intensity is proportional to the half-life of the radioisotope - shorter the half-life the more intense the radiation. Fig. 6 show the radioactive decay chain for thorium 232. All isotopes of thorium has 90 protons and the half-life in this case is 1.4 billion years.
Let's suppose the lens in my Weston 614 according to the literature is 30% thorium oxide, by weight a 1 ounce lens could have a quarter ounce of thorium. Let's argue the lens was made in 1947 and every second we have 10,000 thorium atoms emitting an Alpha particle becoming radium 228 with a half life of 5.7 years. That's every second of every day for the last 70 years. That's over 2.2 BILLION radium atoms and over 2.2 BILLION Alpha particles. This won't even begin to touch the number of atoms of thorium remaining in the lens.
Radium has 88 protons where the thorium lost two protons and two neutrons to the Alpha particle.
But there's even more. Let's just look at the first 10,000 radium atoms from thorium decay - radium 228 has a half-life of 5.7 year. 5.7 years later half of those first 10,000 radium atoms have thrown off a Beta particle (a neutron breaks apart into a proton and electron likely some Gamma radiation as well) becoming Actinium 228 with a half-life 6.1 minutes. 6.1 minutes later half of the Actinium atoms has thrown off another Beta particle.
It's like a radioactive avalanche effect where that single thorium atom in 1947 nine or more Alpha and Beta particles are thrown off in the next century by the time it becomes stable lead 208 (82 protons). This chain starts again every second and they all add together.
Of the Alpha particles are produced in the decay chain most never penetrate the glass while Beta and Gamma radiation passes through. That's why my paper shield has little effect on the lens while totally stopping the Alpha radiation from the smoke detector Americium-241.
Over the decades the glass turns brown.
See the links below for more aspects on this subject.
- Why we should not fear nuclear power.
- Nuclear Graveyards Abound with Life
- What About Humans and Nuclear Radiation?
- Radiation Basics They Should Teach in High School
- Astable CD4047 High Voltage Power Supply
- Geiger Counter and Radioactivity
- Introduction to Geiger-Mueller Counters and Electronics
- CD4047 Monostable Multivibrator Circuit
- Getting Real About Radiation Myths and Hazards
- Uranium Hype-Facts and Virginia Uranium
- Uranium Basics and Isotopes
- Climate Change and Volcanoes
- Geiger Counter Adventures in Radioactivity Literature
- Using TL431A Li-Ion Battery Charger Tutorials
- TL431A Lithium-Ion Cell Charging Circuits
- Charging Multi-Cell Lithium-Ion Battery Packs
- TL431 Over-Voltage, Under-Voltage Detector Circuits
- TL431A Constant Current Source Working Circuits Demo
- Arduino Measures Current from Constant Current Source
- Constant Current Source Theory Testing
- Arduino Controlled Power Constant Current Source
- LM317 Adjustable Current Boost Power Supply
- Constant Current Circuits LM334, LM317
- Build LM317 0-34 Volt Power Supply
- LM334 Constant Current Source with Resistive Sensors
- LM317 High Power Constant Current Source Circuit
- LM317 Constant Current Source Circuits
- Test SCRs and Triacs
- Basic MOSFET Transistor Test Circuits
- High Voltage MOSFET Switching Circuits
- 3 Amp LM741 Op-Amp Constant Current Source
- Current Limiter Testing of Zener Diodes
- Current Limiter for Opto-Coupler Inputs
- LM317 CCS for Light Emitting Diodes
Web site Copyright Lewis Loflin, All rights reserved.
If using this material on another site, please provide a link back to my site.